

(The Amazing World of ElectronsĪ photon of the same energy is emitted when

Photon depend on the energy difference between the orbits:Įnergy "hits" an atom, it can be absorbed and cause an electron to jump to an Lower energy orbit, energy is released in the form of photon. If an electron moves from an outer, higher energy orbit to an inner, Įlectron transitions between energy levels lead to absorption orĮmission of photons of specific energy corresponding to the energy level difference. The branch of physics called quantum mechanics. Levels in an atom because wave-particle duality means they interfere with The nucleus of the atom are held together by the "strong force", which isĬlearly much stronger than the electric one but works only over very small distances. This force attracts positive and negative electricĬharges, but repels like charges - two positives or two negatives. The electrons in an atom are held by the electric force, which is proportional toġ/r 2 just like gravity. With up to about 100 protons, giving 100 elements, each with distinct
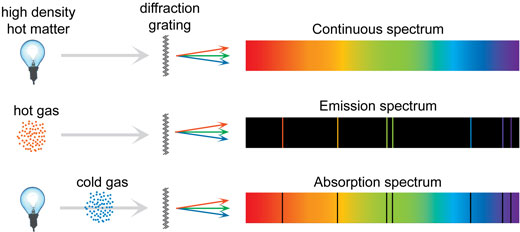
Number of protons and electrons there are atoms Most aspects of the behavior of an atom (e.g., in chemistry) depend just on the Hydrogen (1 proton) and helium (2 protons) are the simplest. Gases tend to have more complex emission- and absorption-line spectra,Īllowing us to learn a lot about their conditions.Įmission- and absorption-line spectra are produced by atoms (and molecules)Ītoms consist of nuclei made of protons and neutrons, and electronsĪround them. In normal experience, solids and liquids tend to emitīlackbody spectra or spectra close to blackbodies. Is a particular type of continuous spectrum.


 0 kommentar(er)
0 kommentar(er)
